FDA allows Houston cancer doctor to resume drug trial
Por um escritor misterioso
Descrição
Federal regulators have lifted a partial hold on a clinical trial performed by Stanislaw

Pralsetinib for RET fusion-positive non-small-cell lung cancer (ARROW): a multi-cohort, open-label, phase 1/2 study - The Lancet Oncology

New Drugs for Ovarian Cancer Patients - WSJ

Harnessing the Immune System to Fight Cancer - The New York Times

Doctor claims to cure pediatric cancer, critics skeptical

Early drug development in solid tumours: analysis of National Cancer Institute-sponsored phase 1 trials - The Lancet
Novartis challenges Pfizer with strong breast cancer drug data
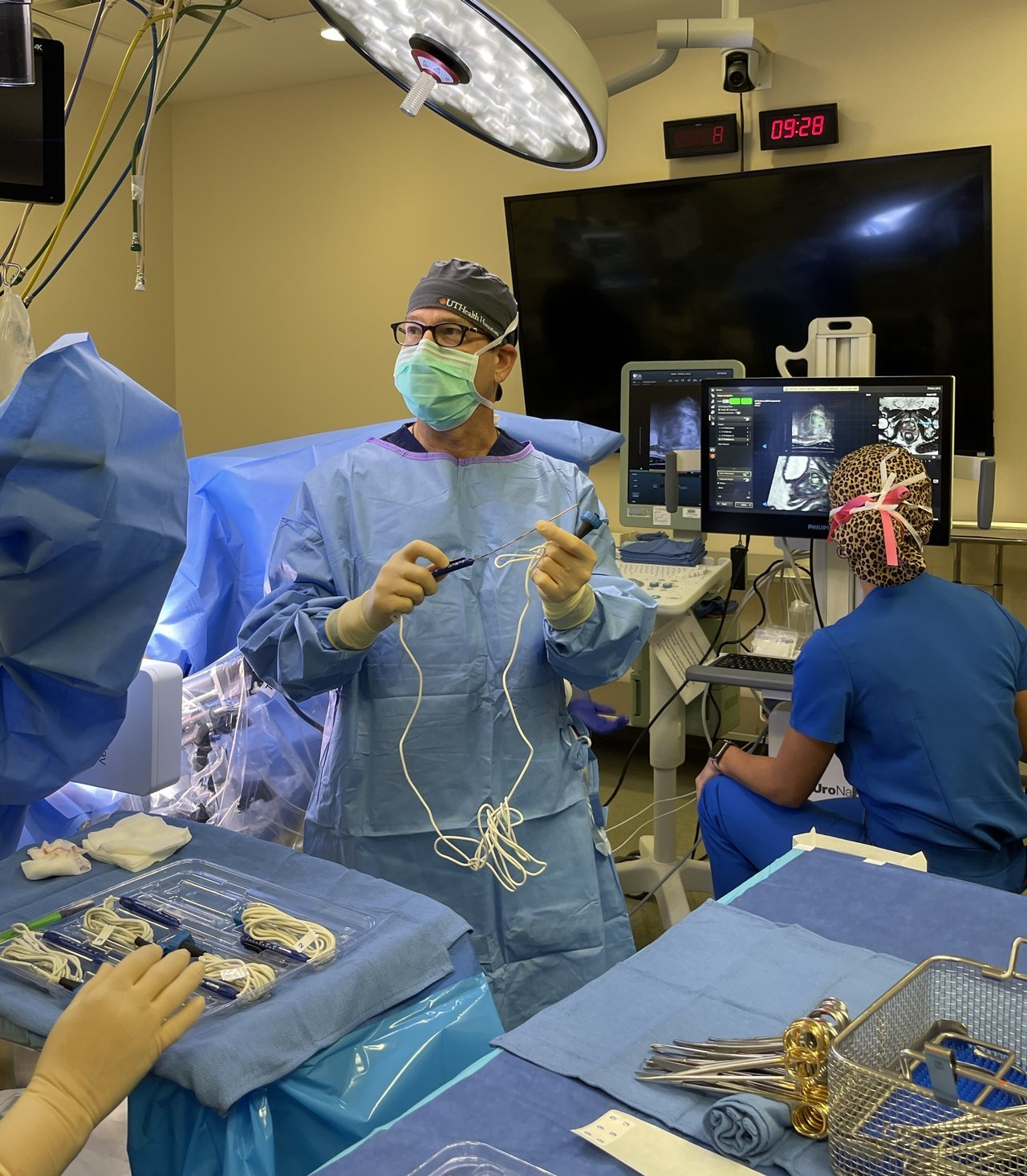
At the Bedside: New focal therapies at UTHealth Houston make prostate cancer treatment easier on patients - UTHealth News - UTHealth Houston

Drug factory' implants eliminate ovarian, colorectal cancer in mice, Rice News, News and Media Relations
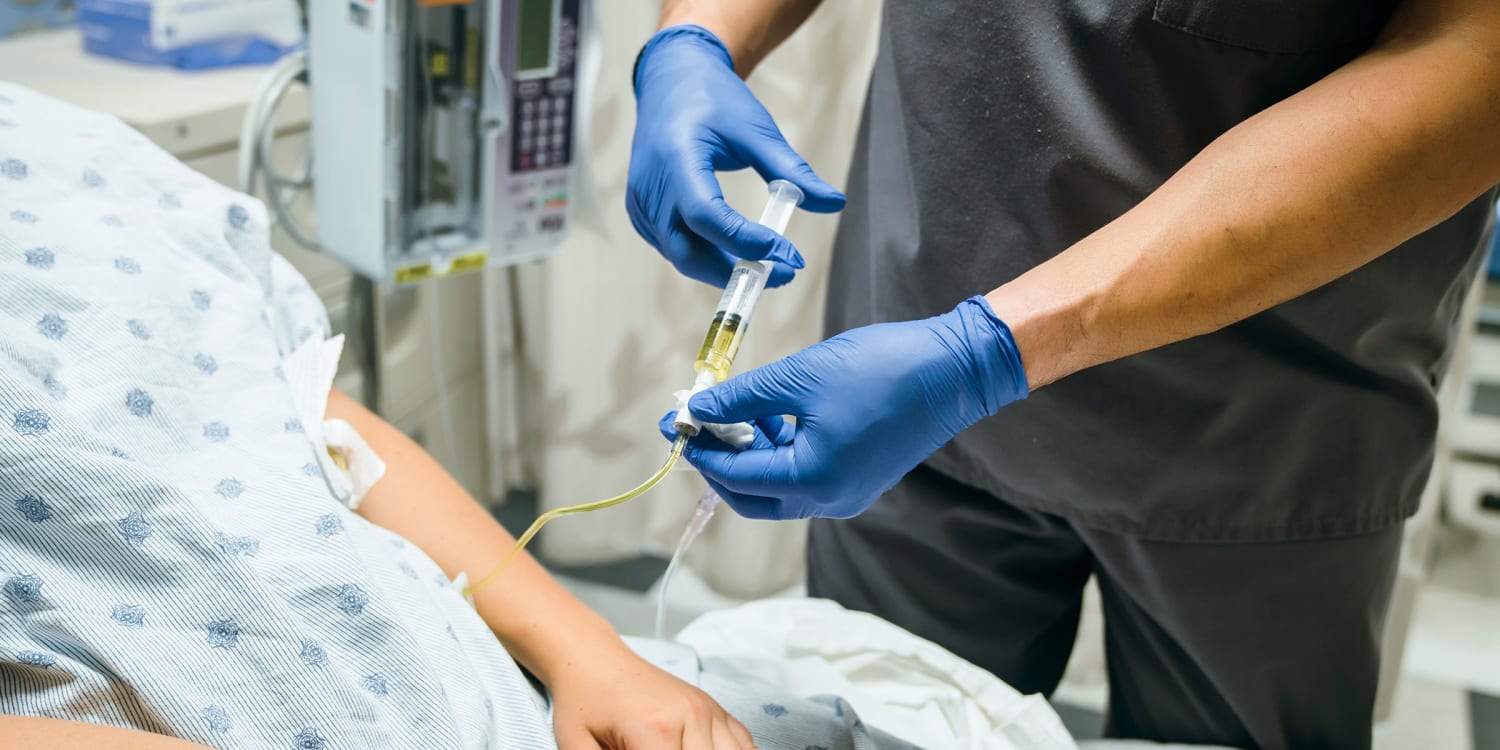
New drug appears to cure rectal cancer in 100% of patients in clinical trial

FDA approves first gene-editing treatment for human illness
In the compelling follow-up to the internationally award-winning documentary, Burzynski, the Movie; Burzynski: Cancer is Serious Business, Part II

Burzynski: Cancer Is Serious Business Part II

FDA panel: Benefit of 'highly anticipated' lung cancer drug can't be interpreted reliably

Brian_Wice
de
por adulto (o preço varia de acordo com o tamanho do grupo)







