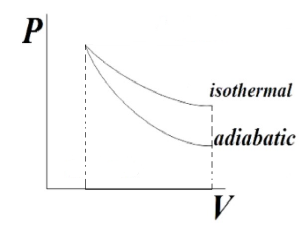
Why is Adiabatic Curve steeper than Isothermal Curve
Por um escritor misterioso
Descrição
Compartilhe seus vídeos com amigos, familiares e todo o mundo
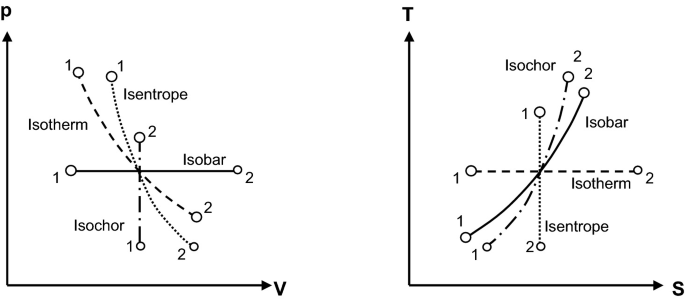
Thermodynamics

Adiabatic curve is steeper than isothermal curve hence magnitude of work done under adiabatic process is more as compared to isothermal process. - India Site

Show that an adiabatic curve is always steeper that an isothermal curve.
In a PV diagram, how do you know the process is isothermal or adiabatic? - Quora
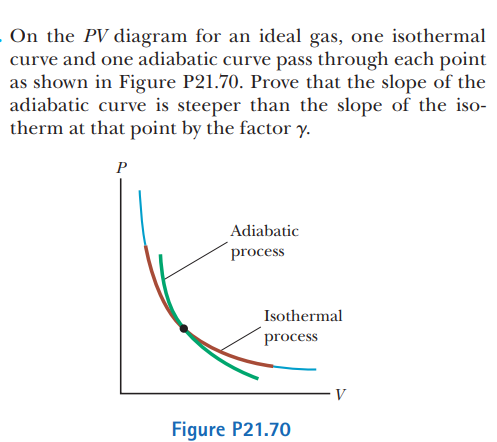
SOLVED: On the P V diagram for an ideal gas, one isothermal curve and one adiabatic curve pass through each point as shown in Figure P21.70. Prove that the slope of the
Explain Graphs -- adiabatic Expansion of mono- di- and poly- gas
The $P-T$ diagram for an ideal gas is shown in the figure, where $AC$ is an adiabatic process. The corresponding $PV$ diagram is

SOLVED: Show that adiabatic curve is steeper than the isothermal curve.

Heat and thermodynamics
Which one among adiabatic and isothermal work done is greater in magnitude and why? - Quora

Bengali] Prove that the adiabatic curves are steeper than the isother

thermodynamics - Why is the curve of an isothermal process above the that of a adiabatic process during compression? - Chemistry Stack Exchange
de
por adulto (o preço varia de acordo com o tamanho do grupo)