Top 4 eConsent Questions in Clinical Research: Forms & More
Por um escritor misterioso
Descrição
Electronic Data Capture Software: 5 Apps for Clinical Research
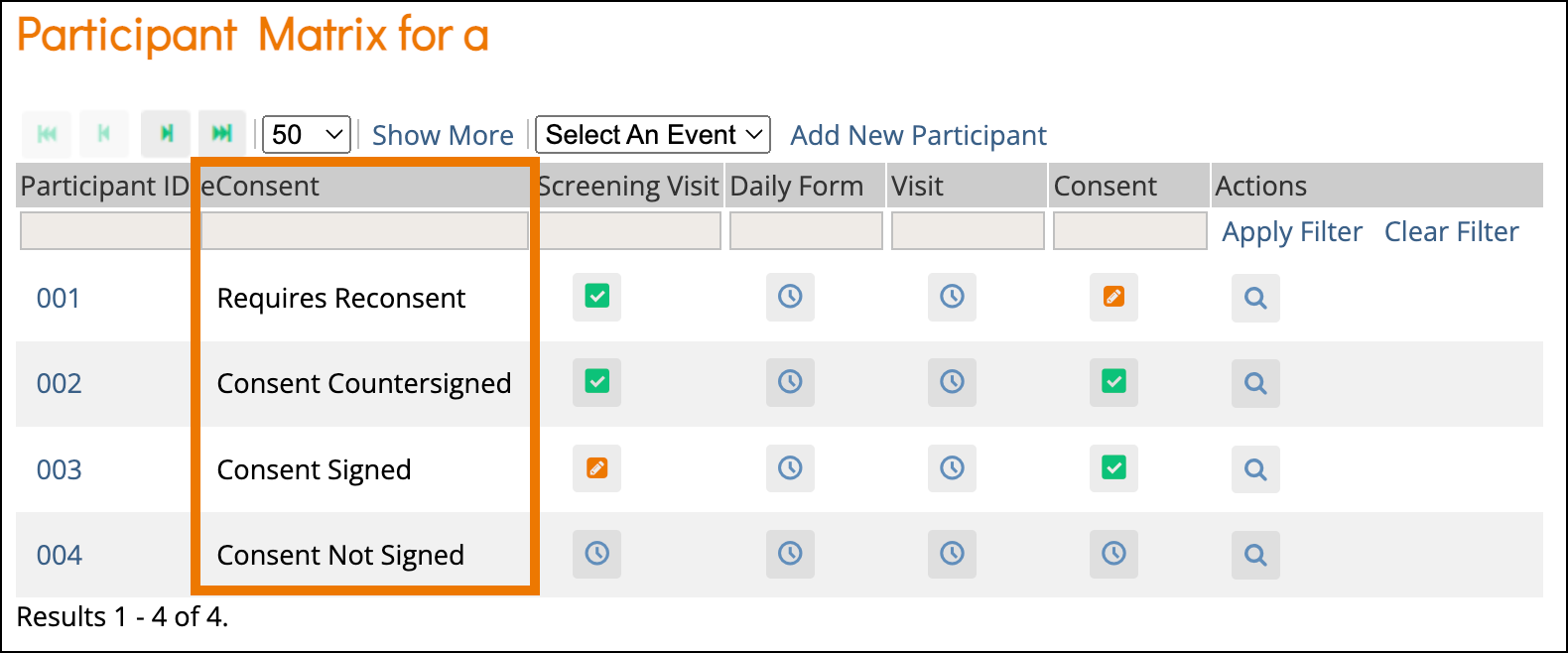
eConsent in Study Runner - OpenClinica Reference Guide
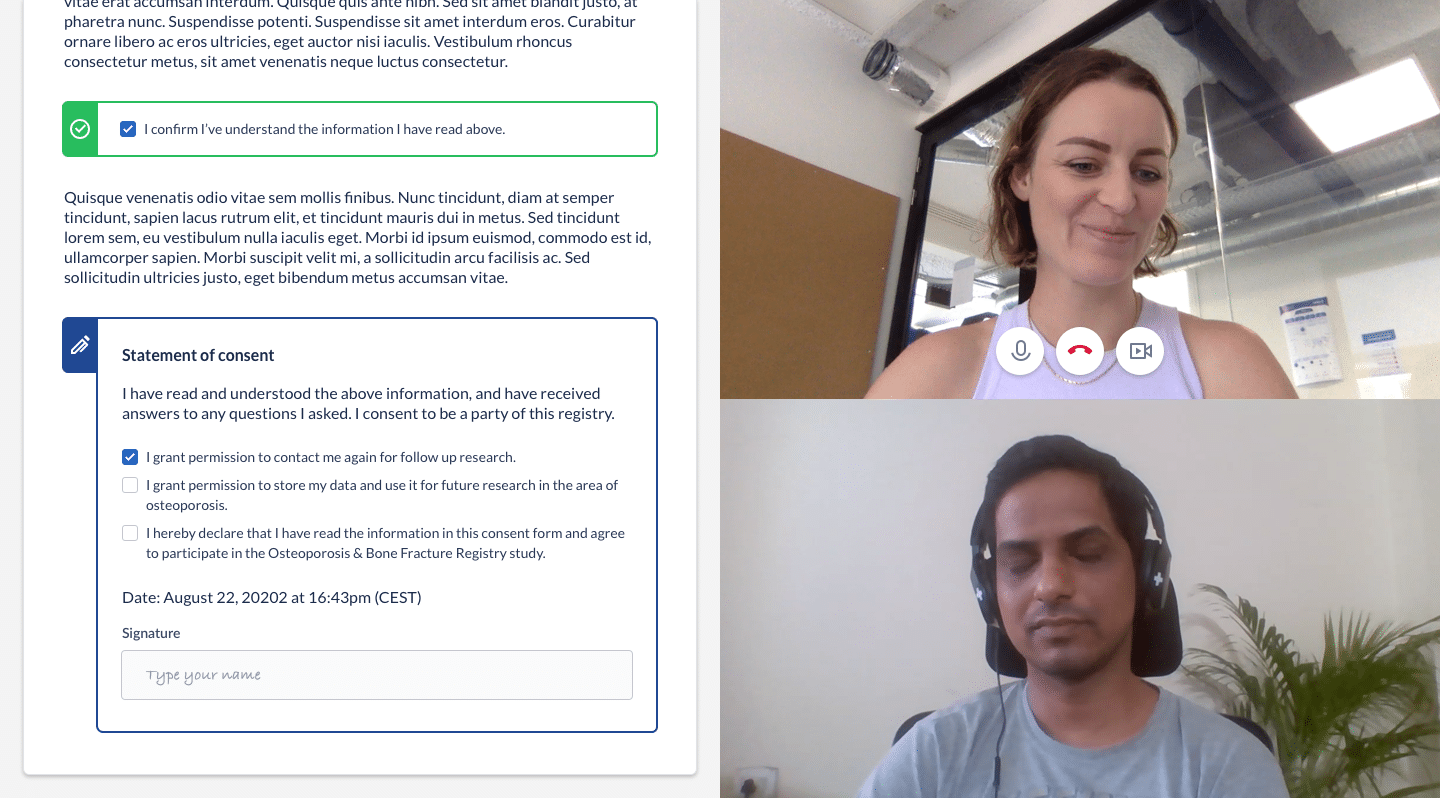
Top 4 eConsent Questions in Clinical Research: Forms & More

E consent for research: Considerations in Implementation and IRB

E-Consent for Clinical Trials - Best Practices & Common Mistakes

Data collection in clinical trials. The typical approach involves

GCP Questions, FDA Answers, 2022 Edition

IRB Approval for eConsent: What to Include in Your Protocol - Vibrent

E-Consent—a guide to maintain recruitment in clinical trials

Awareness and Collaboration Across Stakeholder Groups Important

Using e-Consent Forms in Your Clinical Research

Econsent Solutions: A Better Way to Process Informed Consent in
de
por adulto (o preço varia de acordo com o tamanho do grupo)







